Caudal
Indications: Children (under 12yo or 50kg) undergoing lower abdominal, pelvic, perineal, or lower limb surgeries covering regions between T10 and S5.
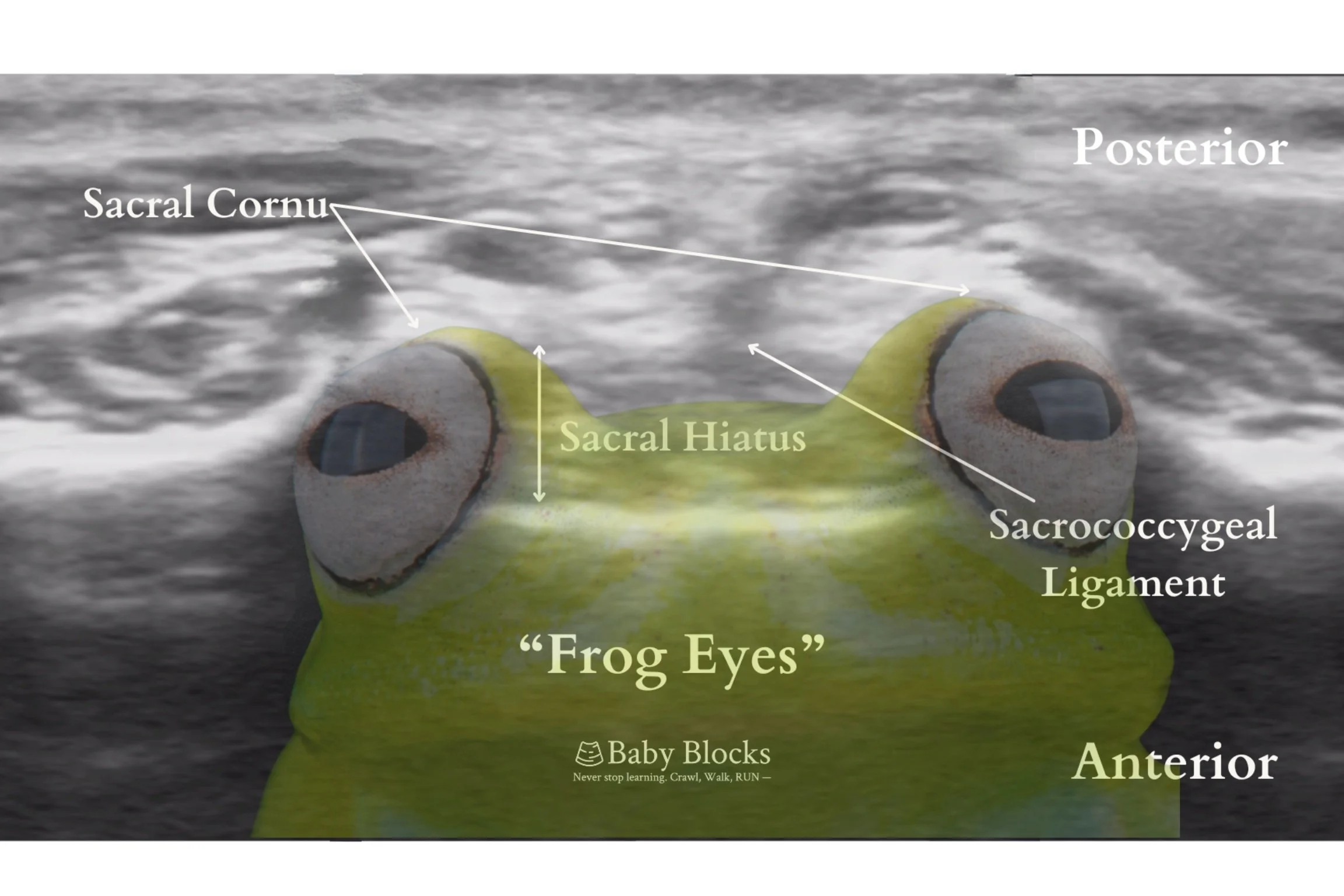
Anatomy: The sacral anatomy is the primary area of interest, as it contains the sacral hiatus, which provides access to the epidural space. The sacral hiatus, triangular in shape, is bordered laterally by bony prominences—remnants of the inferior articular processes of S5 better known as the sacral cornua. It is covered by the sacrococcygeal ligament.
The sacral canal is comprised of the following: the terminus of the dural sac and filum terminale; the sacral and coccygeal nerve components of the cauda equina; the epidural fat (becoming more dense with age and affecting the spread of local anesthetic); the sacral epidural venous plexus (which typically ends at S4). With increasing age, the ligaments and cornua thicken making the sacral hiatal margins difficult to discern.
The infant spinal cord typically ends at the L3/L4 vertebral level whilst the dural sac is known to terminate lower than adults at S3/S4. Of note, there is a great deal of anatomical variability amongst children which can be better defined via ultrasound. Shin et al. found that the dural sac ended lower than S2-S3 in 8% of children in their observational study. Koo et al. ascertained that lateral placement of patients with their neck, hips, and knees maximally flexed correlated with cephalad shifting of the dural sac.
Technique:
Topical Anesthesia: Topical lidocaine or EMLA may be applied to the intended puncture site.
Respiratory Considerations: Spontaneous respiration should be maintained throughout the procedure to minimize airway manipulation. IV access should be established, and adequate sedation may include midazolam (administered orally or rectally) and/or sevoflurane.
Monitoring for Complications:
Breathing patterns may provide early signs of respiratory depression due to systemic absorption of local anesthetic.
Tachypnea may indicate pain on injection.
Tachypnea followed by bradypnea could suggest intrathecal injection of local anesthetic.
Preparation:
The skin should be prepped using a sterile technique with chlorhexidine.
Ultrasound guidance is recommended, using a high-frequency linear transducer (6–15 MHz) due to the superficial location of key structures.
Positioning:
Lateral decubitus (most common) – preferred for easier airway access. The neck and knees should be flexed toward the chest.
Prone – achieved by placing a bump under the pubic symphysis and iliac crests to facilitate hip flexion, or by flexing the knees toward the chest.
Needle type: 22-25 gauge short-bevel Tuohy or Crawford needle, or a 22G angiocath.
Injection Technique:
A 30 cm extension tubing may be attached to the angiocath for local anesthetic injection—ensure the tubing is primed to account for volume. Alternatively, the injectate syringe may be attached directly to the needle hub.
A slow aspiration test using a 3-mL syringe should always be performed before injecting local anesthetic to assess for CSF or blood. Additionally, opening the system to air can help detect free flow.
A test dose of epinephrine (0.5 mcg/kg) injected into the caudal space while monitoring ECG for a >25% increase in T-wave amplitude can help detect intravascular injection.
Confirmatory Tests:
Auditory Confirmation Tests: A specific auditory confirmation test, “whoosh” test, has been described by auscultating the thoracolumbar region during injection of air into the caudal space. The “swoosh” test replaces saline or local anesthetic instead of air as the injectate. It provides a simple, non-invasive adjunct for confirming caudal block placement in children, with high specificity and positive predictive value, but remains subjective and is now considered inferior to ultrasound-based confirmation.
Loss of Anal Sphincter Tone: Loss of anal sphincter tone is highly sensitive for detecting a successful caudal block. (Dave)
Heart Rate Response (Bradycardia): A drop in heart rate ≥ 3 BPM was historically a highly reliable intraoperative predictor of caudal block success under halothane and isoflurane anesthesia; however, its performance is inconsistent under modern sevoflurane-based anesthesia
Epinephrine Test Dose: An epinephrine test dose is a valuable adjunct for detecting inadvertent intravascular injection during caudal block, but it does not predict block efficacy or quality. It’s utility is limited under general anesthesia, where Walker et al. found it to have a high positive predictive value but a limited negative predictive value. For this reason, the test dose should complement—rather than replace—other methods such as ultrasound guidance.
Fluoroscopy: Fluoroscopy is the gold standard for confirming catheter placement but is rarely used due to radiation exposure and impracticality.
Ultrasound: Ultrasound allows real-time visualization of needle placement and local anesthetic spread during caudal block. Ultrasound-guidance improves first-pass success and reduces complications such as vascular puncture and subcutaneous injection, and prospective data from over 100 patients showed it identified ~20% of landmark-based placements as incorrect. However, its impact on overall clinical outcomes remains uncertain. A meta-analysis by Jain et al. found no significant difference in block success rates or procedure time compared to landmark techniques. The largest prospective study of landmark caudals by Suresh et al (n = 16,342) reported a 1% failure rate, suggesting high reliability even without ultrasound. While ultrasound enhances precision, its superiority in safety or efficacy remains unproven.
Summary: Adjunctive tests such as HR change, the swoosh test, and epinephrine test dose offer adjunctive confirmation of caudal block. However, ultrasound now provides the most reliable method—improving first-pass success, reducing complications, and identifying landmark-based errors—while overall block success remains high (~95–96%) across all techniques (landmark or ultrasound-guided).
Landmark Technique
The sacral hiatus is identified by palpating the posterior superior iliac spines (PSIS), with the line between these spines forming the base of an equilateral triangle (pointed caudally). The apex of this triangle lies over the sacral hiatus between the sacral cornua. Palpation of the sacral hiatus as a bony landmark is typically located at the level of the sacrococcygeal joint. The thumb may be used to palpate the two sacral cornua at the upper end of the intergluteal cleft.
After confirming the anatomical landmarks, the needle is inserted at a 45-degree angle in a caudal-to-cranial direction. A distinct “pop” may be appreciated as the needle traverses the sacrococcygeal ligament, followed by a loss of resistance. At this point, the angle of the needle should be decreased, and the needle advanced an additional 2–3 mm to facilitate easy catheter threading. Continuous palpation of the area is recommended to detect subcutaneous bulging, which may indicate improper placement.
In neonates, identification of the sacrococcygeal membrane by palpation alone can be challenging due to incomplete ossification of the posterior vertebrae. Mirjalili et al. found that the equilateral triangle method may be unreliable when compared to ultrasound confirmation of actual anatomical landmarks.
Ultrasound-Assisted Technique
An ultrasound-assisted technique involves an initial ultrasound assessment of relevant anatomical structures before performing a landmark-based caudal puncture. This preliminary evaluation allows for a more precise mapping of the caudal anesthetic procedure and may help bypass the steeper learning curve associated with simultaneously manipulating the probe and performing the procedure. Following the administration of the local anesthetic, ultrasound can be used at the end to confirm proper placement of the injectate within the caudal space. While this may enhance procedural confidence, current evidence has not established a correlation between ultrasound-observed spread and clinical block effectiveness. Moreover, post-injection imaging reflects only immediate distribution and may underestimate full cephalad spread due to delayed migration of anesthetic.
Ultrasound-Guided Technique
As a big proponent of using all your applicable senses when performing a procedure to ensure safety and success, it makes sense (no pun intended) to supplement visual confirmation of the caudal placement with tactile perception. Ultrasound facilitates needle tracking for precise localization and access to challenging caudal spaces, while also enabling dynamic flow tracking for adequate local anesthetic spread to the desired level.
Probe placement begins with a transverse (or short axis) approach over the coccyx, scanning cephalad to identify key hyperechoic structures. These include the sacrococcygeal ligament, appearing as a band between the two cornua (the “frog-eye sign”), and the deeper dorsum of sacral bone. As the probe moves cephalad, structures such as the the dural sac termination, cauda equina fibers, filum terminale, and conus medullaris may be visualized. The optimal probe position is near the apex of the triangular area formed by the sacral cornua and tip of the dorsal sacrum, where the caudal space is the widest. Within this space, between the ligament and sacral bone, the sacral hiatus appears hypoechoic, marking the ideal insertion site for the needle. Kollipara et al. foud that the optimal angle of needle insertion in ultrasound-guided blocks was 20-25 degrees, aligned parallel to the sacral base.
Transverse or Short Axis, aka “Frog Eyes” View
Next, the transducer is rotated 90° to obtain a longitudinal (sagittal) view, with the needle in-plane to the sacral hiatus [Figure 4]. In this orientation, the sacrococcygeal ligament appears as a hyperechoic line extending from inferior S3 to the distal sacrum, covering an anechoic caudal space. As the needle is advanced into the caudal space, local anesthetic is injected, and dynamic flow tracking can be observed, with anterior displacement of the dura mater serving as confirmation.
Sagittal or Long Axis
When teaching the ultrasound-guided approach to other skilled practitioners, a common challenge noted was difficulty stabilizing the transducer probe during the caudal block, as there was a tendency for the probe to slide off the spinous process and away from midline. To improve stability, spreading the fingers while applying pressure on the OR table can help steady the hand. Another technique involves holding the probe close to the scanning edge, allowing the proceduralist to stabilize their hand against the patient’s back. Alternatively, a three-hand approach has been described, in which an assistant holds the ultrasound probe while the proceduralist focuses on needle placement.
It’s the first block many of us learn—and still the most globally used pediatric regional technique. The caudal block has outlived trends, adapted to new tools, and proven itself across settings and patient populations. It’s fast. It works across ages. It can be performed with basic positioning and minimal setup. And when done well, it delivers consistent, dense analgesia from the perineum to the lower abdomen. Its pediatric history stretches back nearly a century: Meredith Campbell’s 1933 report was likely the first to describe caudal anesthesia in children, noting its success in avoiding general anesthesia for urologic instrumentation. Later popularized in modern pediatric anesthesia by Dr. Estela Melman and others, it remains unmatched in global reach and clinical utility.
The data confirm what clinicians already know. PRAN’s 100,000-block series by Walker et al. reported no permanent neurologic deficits, transient neurologic complications in just 2.4:10,000 cases, and a LAST risk of 0.76:10,000. In 18,650 caudals specifically, PRAN showed a 98.3% success rate. Caudal remains the single most commonly performed neuraxial block in pediatric regional anesthesia worldwide. The 2025 Delphi consensus by Hagen et al. reaffirmed it as one of eight global core techniques for pediatric regional anesthesia. Today, caudal is still the go-to for hypospadias repair, perineal surgery, lower abdominal surgery, and neonatal operations where neuraxial analgesia offers clear advantages. This isn’t a legacy block—it’s one of the few techniques that scales from basic to expert practice, across neonates, infants, and older children.
Confirmation tools have evolved. Heart rate fall ≥3 bpm , swoosh test (Orme 2003), and epinephrine test doses remain useful—especially where ultrasound isn’t available—but each has limitations. HR fall is less reliable under sevoflurane, swoosh is subjective, and test dosing is excellent for detecting intravascular placement but doesn’t confirm block efficacy. Ultrasound adds valuable precision and safety. It improves first-pass success, reduces minor complications, and identified ~20% of landmark attempts as misplacements. Landmark technique remains highly effective—with 98.3% success in PRAN and ~95% in controlled trials—but ultrasound offers an added layer of precision and confidence.
Ultrasound has also reshaped how we think about dosing and spread. A dynamic flow study suggest that 1 mL/kg reliably reaches T12–L1, with diminishing cephalad spread as children age. In neonates and patients with sacral anomalies, ultrasound provides meaningful safety advantages—reducing dural puncture risk and helping avoid vascular injury. While epinephrine test dosing has limited negative predictive value under general anesthesia, its high positive predictive value—49 positive tests in single-shot caudals in Walker et al—likely prevented additional toxicity events. The caudal block remains the benchmark for safe, scalable neuraxial practice in children—one that every pediatric regional anesthesiologist should master.
Mirjalili SA, Taghavi K, Frawley G, Craw S. Should we abandon landmark-based technique for caudal anesthesia in neonates and infants? Paediatr Anaesth. 2015 May;25(5):511-6. doi: 10.1111/pan.12576. Epub 2015 Jan 16. PMID: 25597342.
Kim HJ, Kim H, Lee S, Koh WU, Park SS, Ro Y. Reconsidering injection volume for caudal epidural block in young pediatric patients: a dynamic flow tracking experimental study. Reg Anesth Pain Med. 2024 May 7;49(5):355-360. doi: 10.1136/rapm-2023-104409. PMID: 37429622.
Campbell MF. Caudal anesthesia in children. J Urol. 1933;30(2):245–50.
Melman E, Penuelas JA, Marrufo J. Regional anesthesia in children. Anesth Analg. 1975 May-Jun;54(3):387-90. doi: 10.1213/00000539-197505000-00034. PMID: 1169030.
Walker BJ, Long JB, Sathyamoorthy M, et al. Complications in pediatric regional anesthesia: An analysis of more than 100,000 blocks from the Pediatric Regional Anesthesia Network. Anesthesiology. 2018;129(4):721–32.
Suresh S, Long J, Birmingham PK, De Oliveira GS. Are caudal blocks for pain control safe in children? An analysis of 18,650 caudal blocks from the Pediatric Regional Anesthesia Network (PRAN) database. Anesth Analg. 2015;120(1):151–6.
Hagen JG, Kattail D, Barnett N, et al. Baby steps to mastery: building blocks for novices in pediatric regional anesthesia. Reg Anesth Pain Med. 2025;epub ahead of print. doi:10.1136/rapm-2025-106434
Dave NM, Garasia MB. A comparison of the effectiveness of predictors of caudal epidural block success in children. J Anaesthesiol Clin Pharmacol. 2012;28(2):210–4.
Ghai B, Makkar JK, Batra YK, Rao KLN. Is a fall in baseline heart rate a reliable predictor of a successful single shot caudal epidural in children? Paediatr Anaesth. 2007;17(6):552–6.
Orme RM, Berg SJ. The 'swoosh' test--an evaluation of a modified 'whoosh' test in children. Br J Anaesth. 2003 Jan;90(1):62-5. PMID: 12488381.
Adler AC, Belon CA, Guffey DM, et al. Heart rate response to a caudal block in children anesthetized with sevoflurane after ultrasound-confirmed injection. Paediatr Anaesth. 2015;25(10):990–6.
Jain D, Hussain SY, Ayub A. Comparative evaluation of landmark technique and ultrasound-guided caudal epidural injection in pediatric population: A systematic review and meta-analysis. Paediatr Anaesth. 2022;32(1):35–42.
Ponde V, Shah D, Nagdev T, Balasubramanian H, Boretsky K. Ultrasound determination of the dural sac to sacrococcygeal membrane distance in premature neonates. Reg Anesth Pain Med. 2022;47(5):327–9.